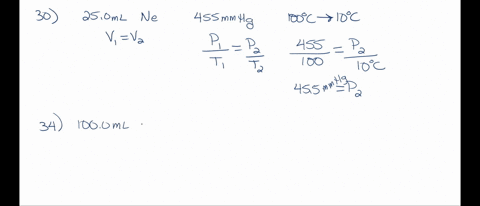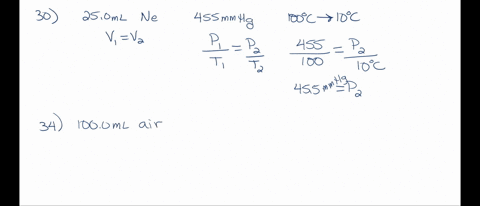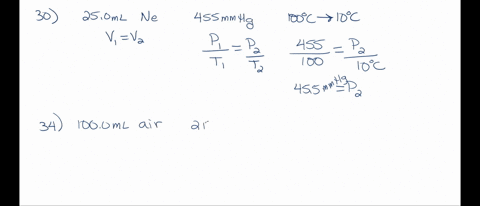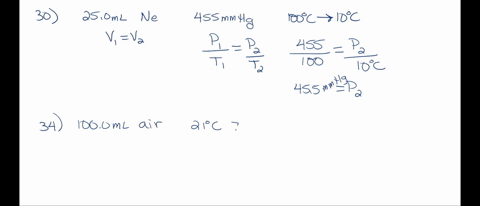
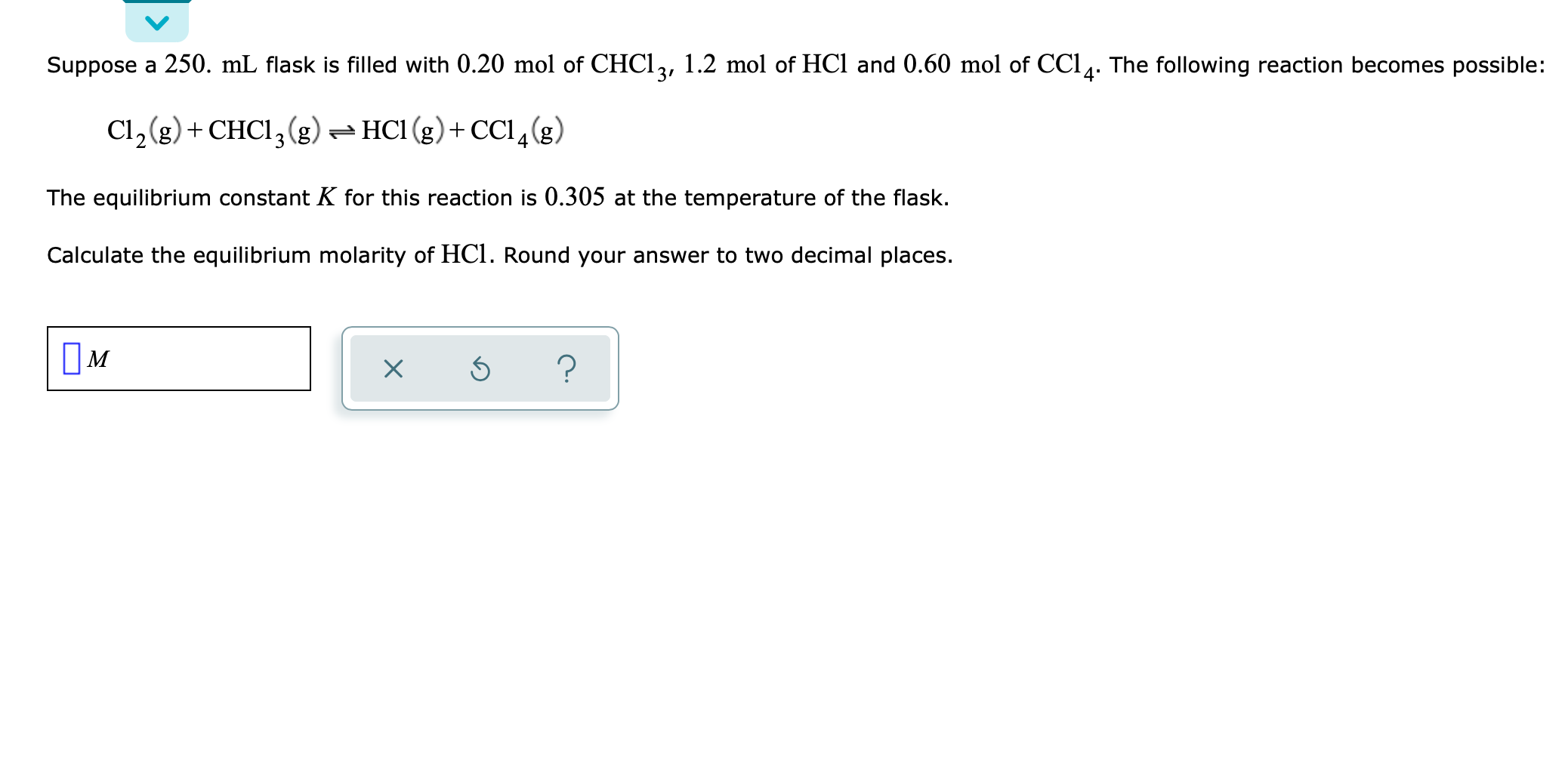
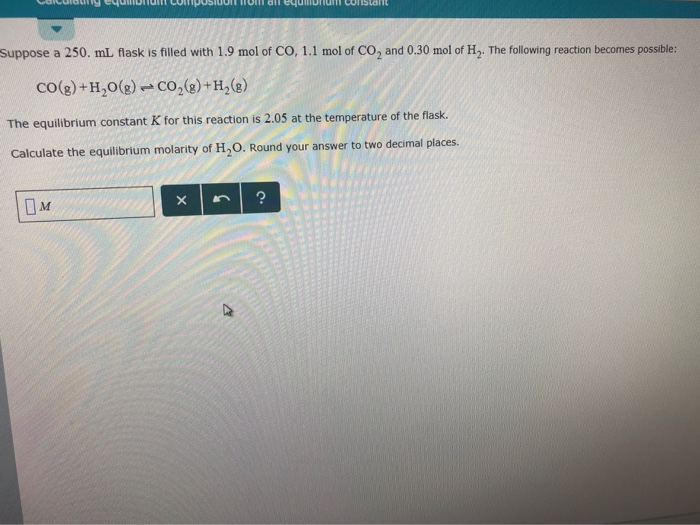
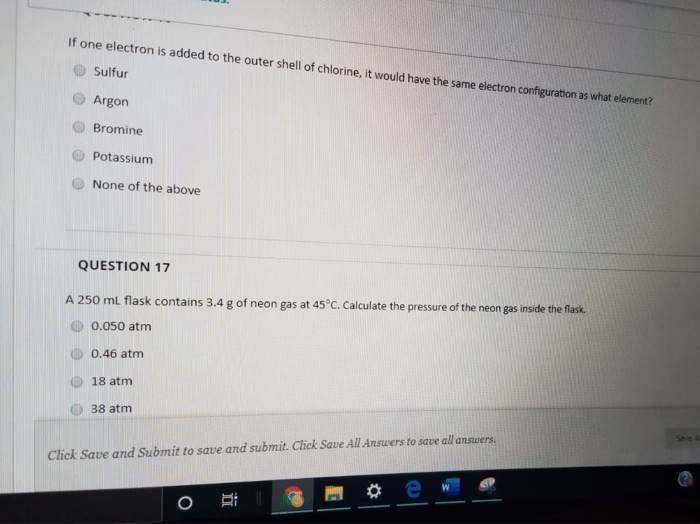
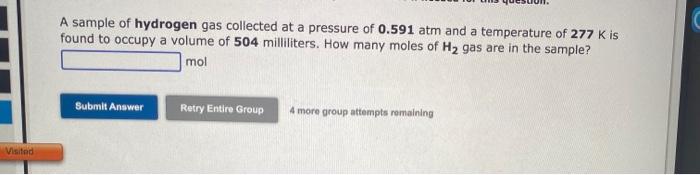
SOLVED: A 250 mL flask contains 54 mol of neon gas at 45°C. Calculate the pressure of the neon gas inside the flask. Options: A. 0.050 atm B. 0.46 atm C. 18

SOLVED: A 250.0 mL flask contains 3.4 g of argon gas at 45.0°C. Calculate the pressure of the neon gas inside the flask: (R = 0.0821 L·atm/mol·K; Use 273.15 for conversion to

A flask contains a mixture of Ne(g) and Ar(g). There are 0.250 mol of Ne(g) which exerts a pressure - YouTube

SOLVED: A 250.0 mL flask contains 3.4 g of argon gas at 45.0°C. Calculate the pressure of the neon gas inside the flask. (R = 0.0821 L.atm/K.mol; Use 273.15 for conversion to K.)