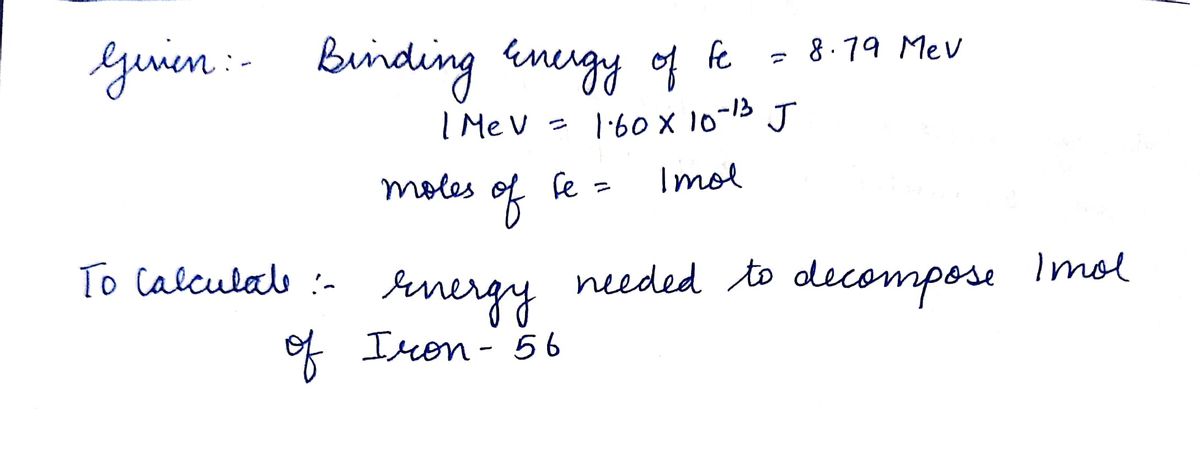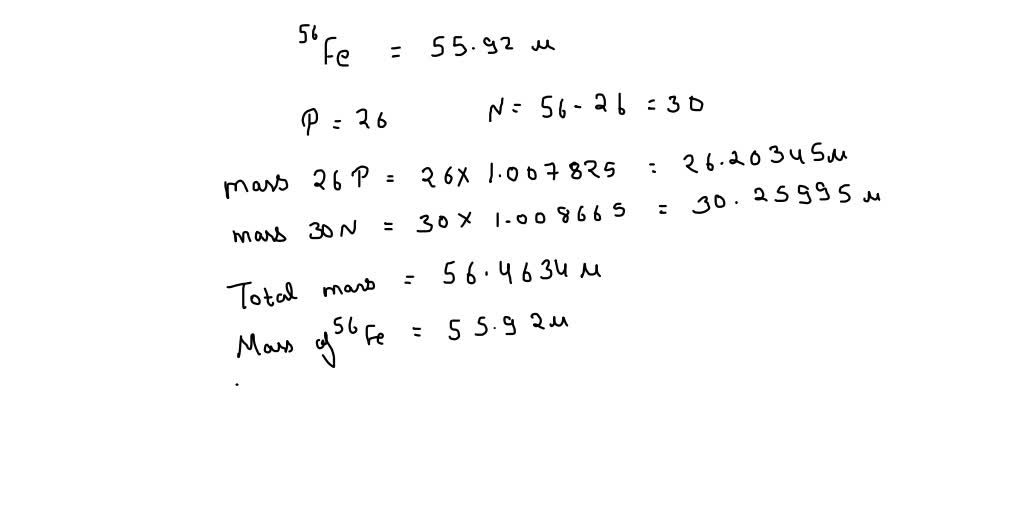
SOLVED: The mass of an iron-56 nucleus is 55.92066 units. What is the mass defect of this nucleus? What is the binding energy of the nucleus? Find the binding energy per nucleon.
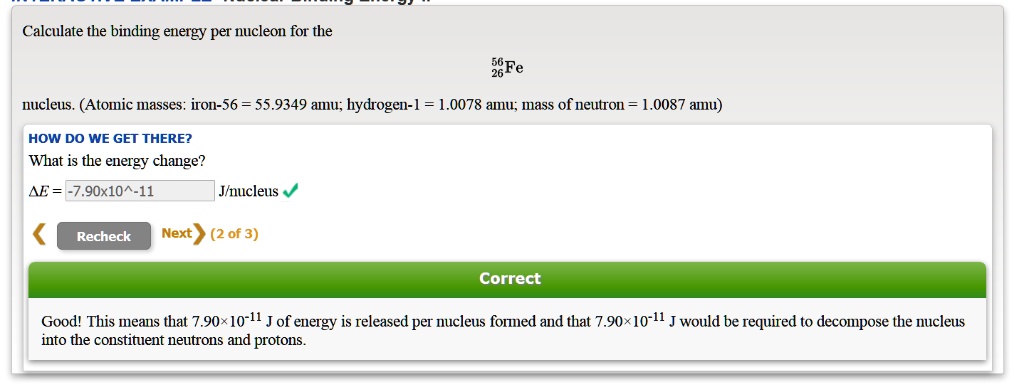
SOLVED: Calculate the binding energy per nucleon for the Fe nucleus. (Atomic masses: iron-56 55.9349 amu; hydrogen-1 = 1.0078 amu; mass of neutron: 1.0087 amu) HOW DO WE GET THERE? What is

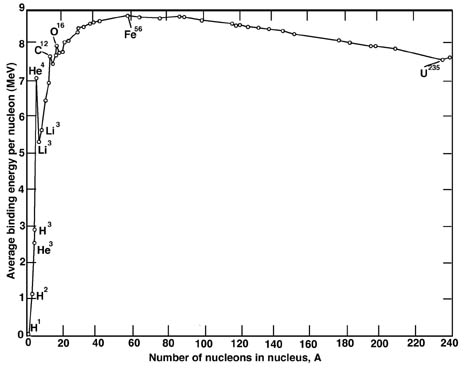
OneClass: Iron-56 (56Fe) has a binding energy per nucleon of 8.790 MeV. (1MeV is 1.602 × 10–13 J)....

Find the binding energy of `_26^56 Fe`. Atomic mass of `^56 Fe` is `55.9349 u` and that of `^1 H... - YouTube
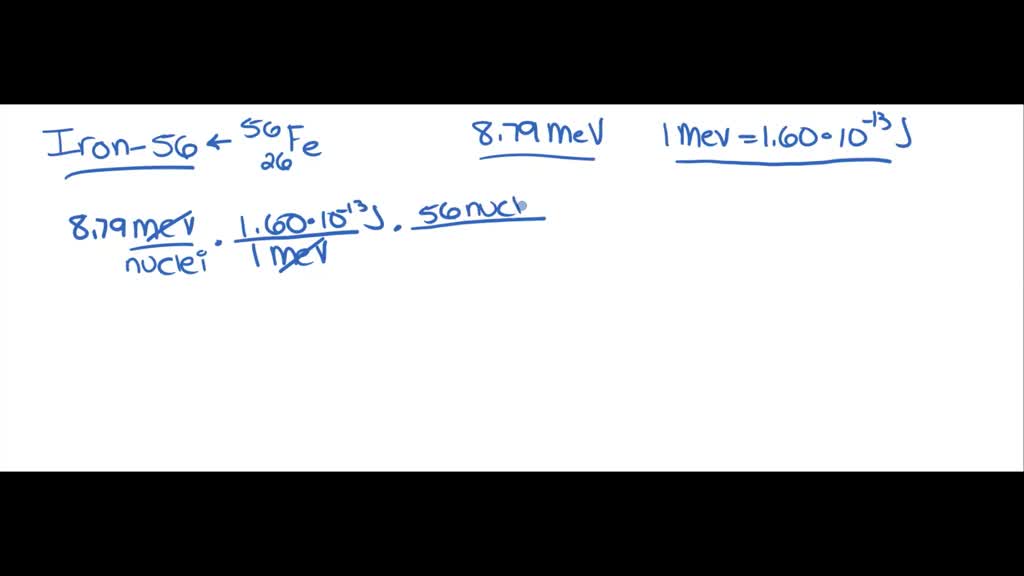
SOLVED: Iron-56, ⠵⠶₂₆Fe, has a binding energy per nucleon of 8.79 MeV (1 MeV = 1.60 × 10⠻¹³ J). Determine the amount of energy needed to 'decompose' 1 mol

SOLVED: The 56Fe nucleus has an experimentally determined mass of 55.9207 amu. The mass of a proton is 1.00728 amu, while the mass of a neutron is 1.00866 amu. What is the
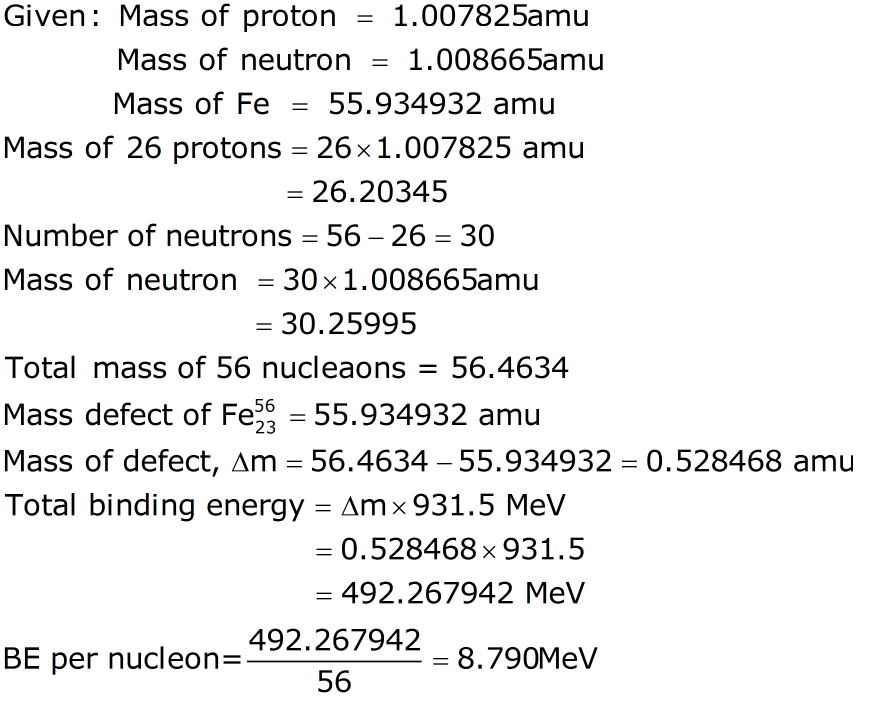
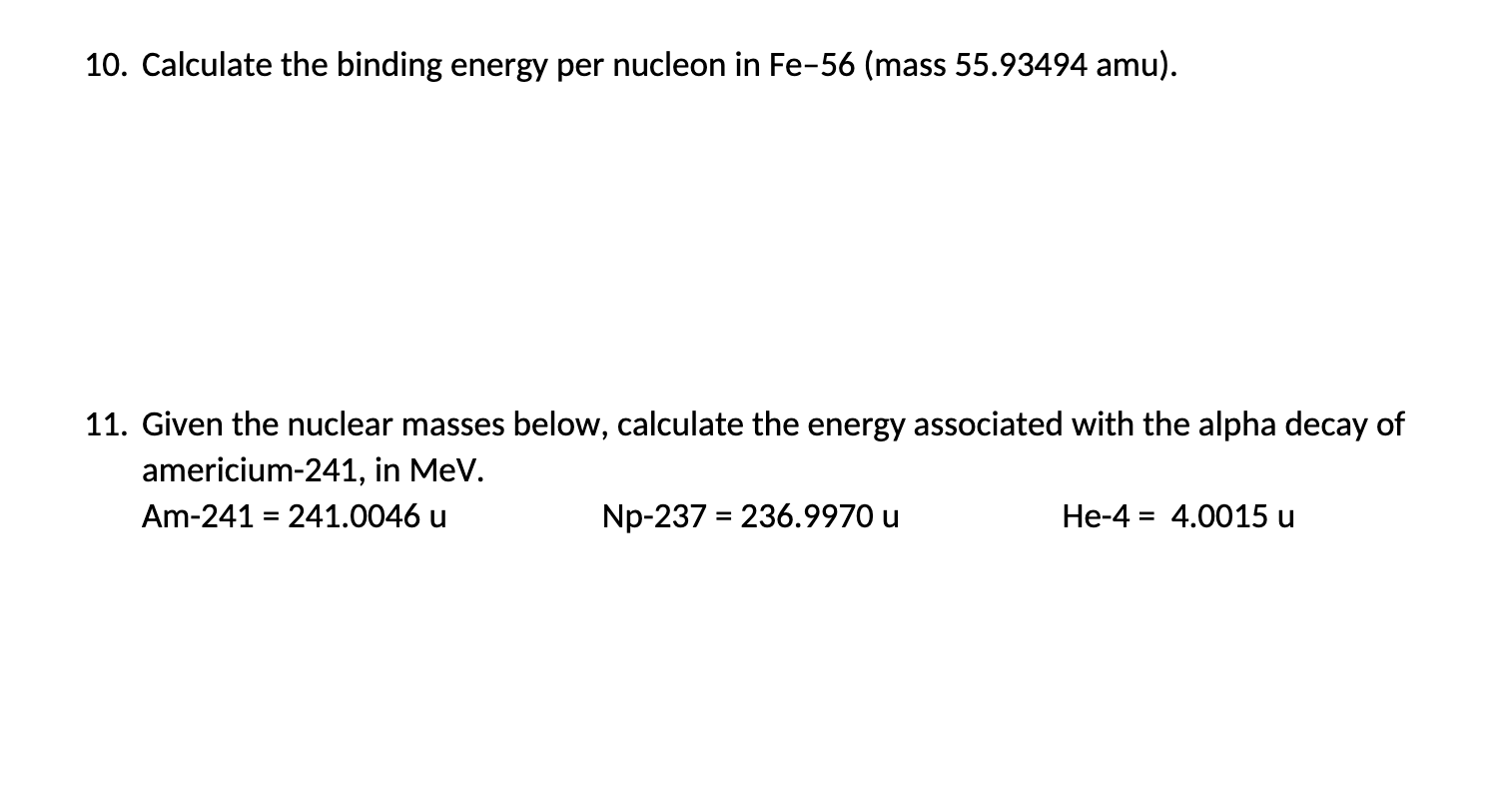
Calculate the binding energy and binding energy per nucleon of 26Fe raise to 56 nucleus. given, Mass of proton = 1.007825amu Mass of neutron = 1.008665amu Mass of Fe = 55.934932 amu - voecphoo
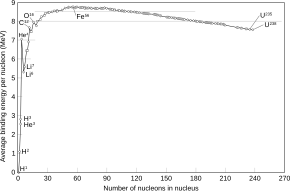
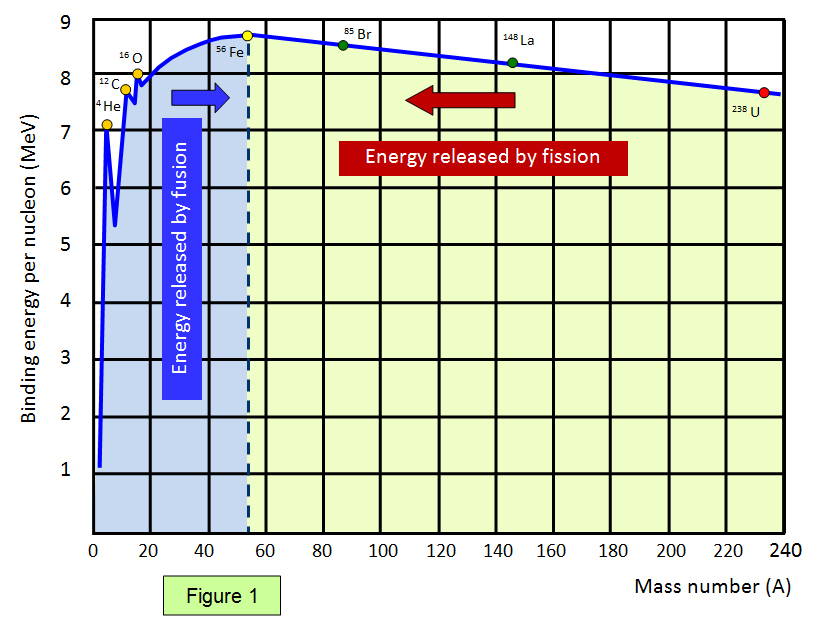
✓ Solved: The most stable nucleus in terms of binding energy per nucleon is ^56Fe. If the atomic mass...
![Calculate binding energy for ^5626Fe nucleus [ Given: mass of ^5626Fe = 55.9349 u, mass of neutron = 1.00867 u, mass of proton = 1.00783 u and 1 u = 931 MeV/c^2 ]. Calculate binding energy for ^5626Fe nucleus [ Given: mass of ^5626Fe = 55.9349 u, mass of neutron = 1.00867 u, mass of proton = 1.00783 u and 1 u = 931 MeV/c^2 ].](https://haygot.s3.amazonaws.com/questions/1967916_1836808_ans_d3b297af78754fa693cc034fa3b7e105.jpg)