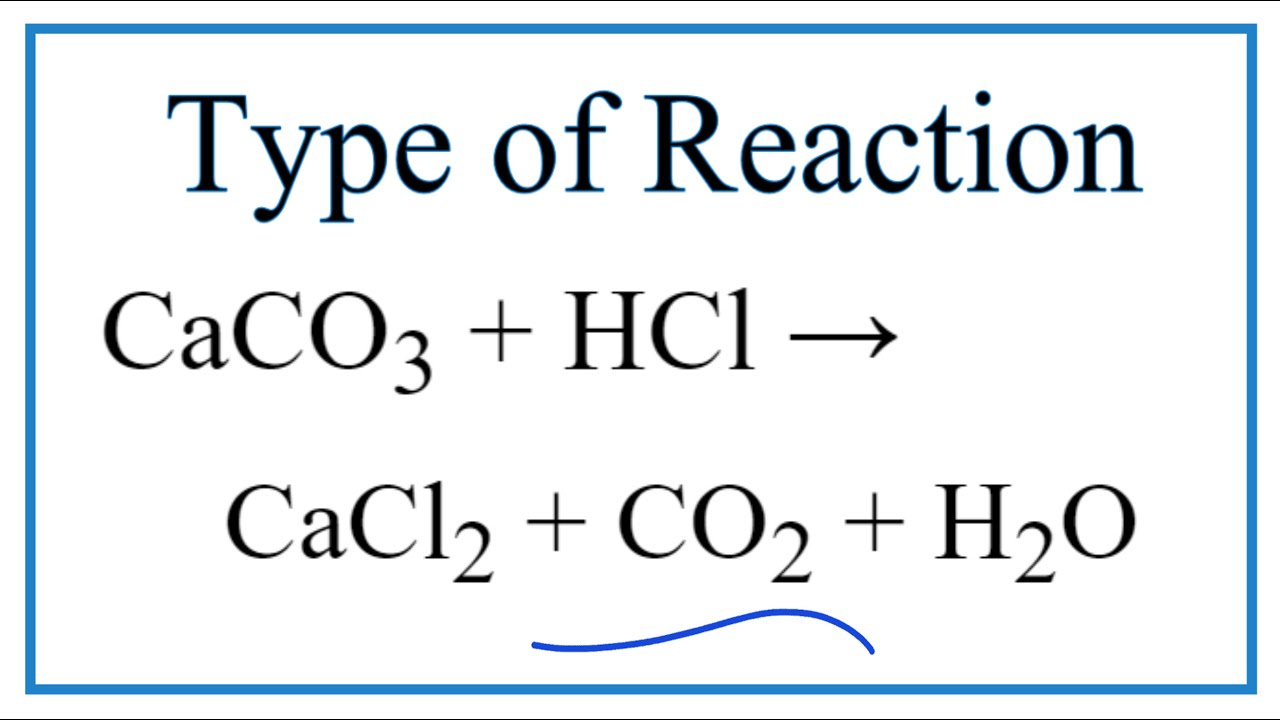
Chemistry: Chemical Word Equations Directions: Write a balanced chemical equation for each of the word equations below. - ppt download
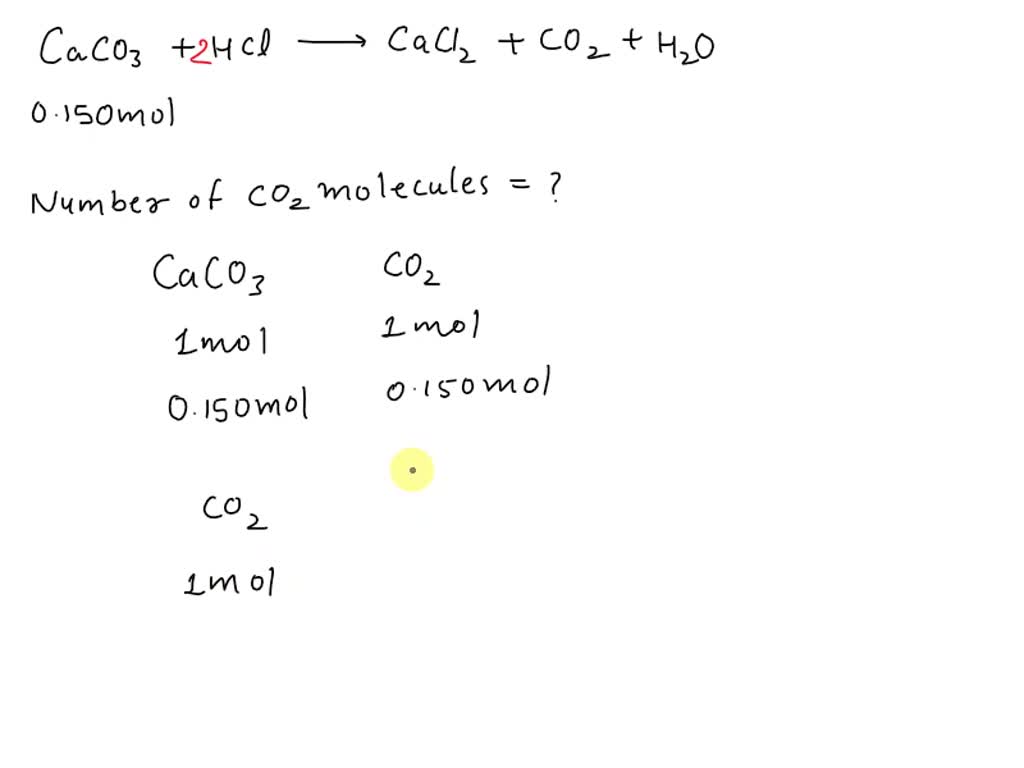
SOLVED: CaCO3+HCl—>CaCl2+CO2+H2O ¿Cuántas moléculas de dióxido de carbono se desprenden al reaccionar 0,150 mol de carbonato de calcio?

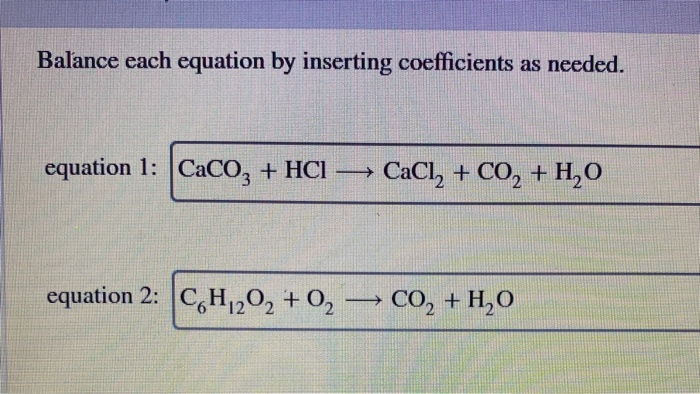
Balance the following equations: i) CaCO3 + HCl → CaCl2 + CO2 ↑ + H2O ii) Na + H2O → NaOH + H2 ↑ iii) (NH4)2 SO4 + Ca(OH)2 → CaSO4 +
16. In a chemical reaction, caco3+2hcl= cacl2 +co2+h2o. 25ml hcl and 0.75M Calculate the amount of caco3
40. Consider the reaction CaCO3+2HCL (l) 》CaCl2+CO2+H2O (l).what mass of CaCO3 is required to react with 20mL 1M HCL?

Balance the following equations : (a) Caco, (s) + HCl (aq) + CaCl, (aq) + H2O (1) + CO, (g) (b) Zn (s) + HCl - Brainly.in
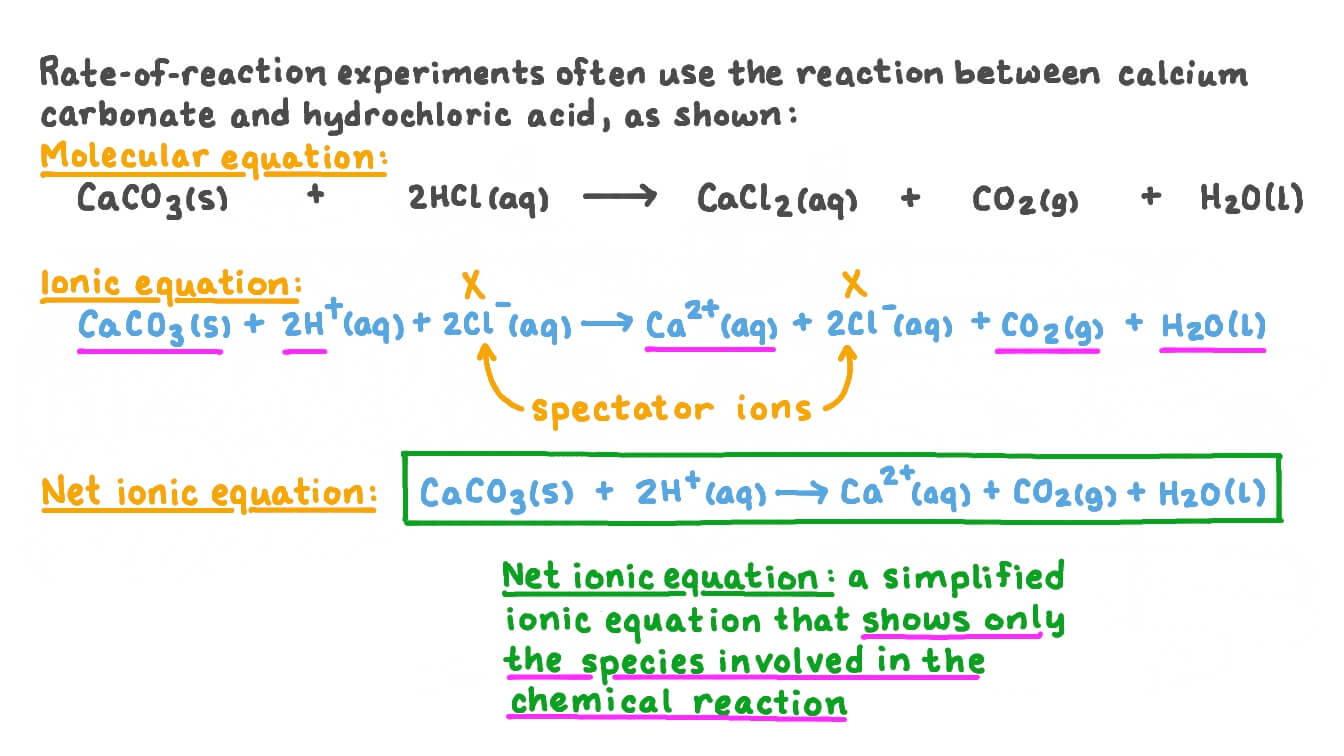
Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

Calcium carbonate react with aqueous HCI to given below: CaCO3(s) + 2HCl (aq) → CaCl2 + CO2 + H2O What mass - Brainly.in

SOLVED: Solid calcium carbonate (CaCO3) reacts with hydrochloric acid (HCl) to form carbon dioxide, water, and calcium chloride (CaCl2), according to this equation: CaCO3(s) + 2HCl(aq) â†' CaCl2(aq) + H2O(aq) + CO2(g)
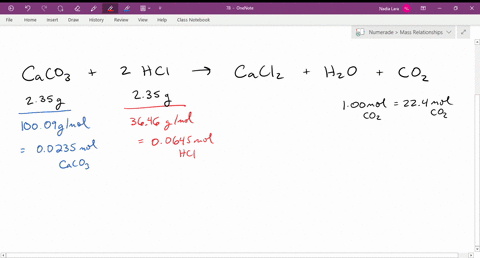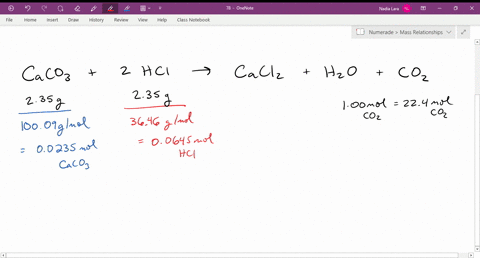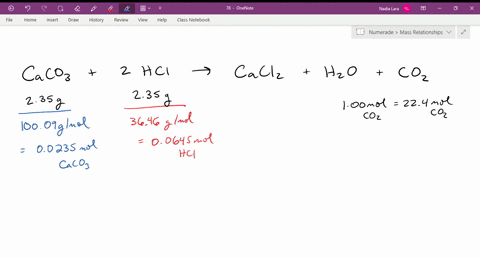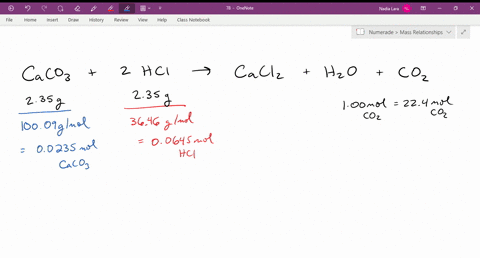
SOLVED: The reaction of limestone with hydrochloric acid is: CaCO3 + 2HCl ⟶ CaCl2 + CO2 + H2O If the reaction produced 26.5 g of CO2, how many grams of HCl reacted?

Question Video: Calculating the Average Rate of Reaction of Hydrochloric Acid with Calcium Carbonate | Nagwa
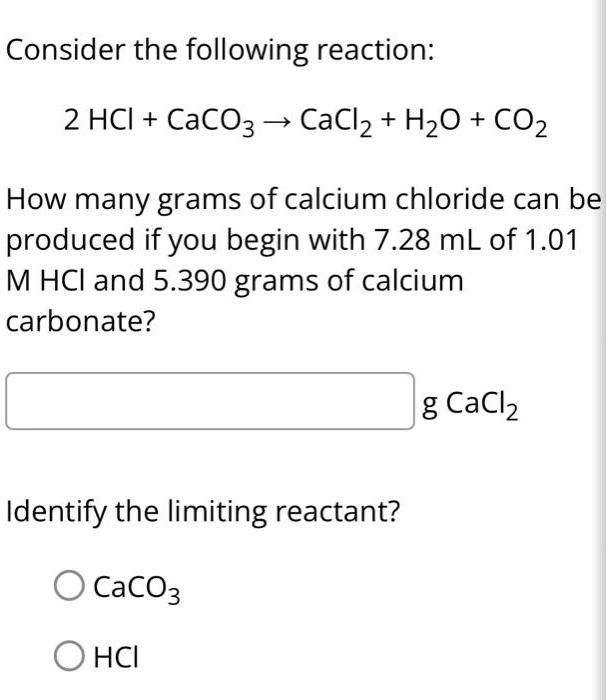
SOLVED: Consider the following reaction: 2 HCl + CaCO3 -> CaCl2 + H2O + CO2 How many grams of calcium chloride can be produced if you begin with 7.28 mL of 1.01

CaCO3+HCl=CaCl2+CO2+H2O Balanced Equation||Calcium Carbonate+Hydrochloric acid Balanced Equation - YouTube

HCl+CaCO3=CaCl2+H2O+CO2 balance the chemical equation @mydocumentary838. hcl +caco3=cacl2+h2o+co2 - YouTube