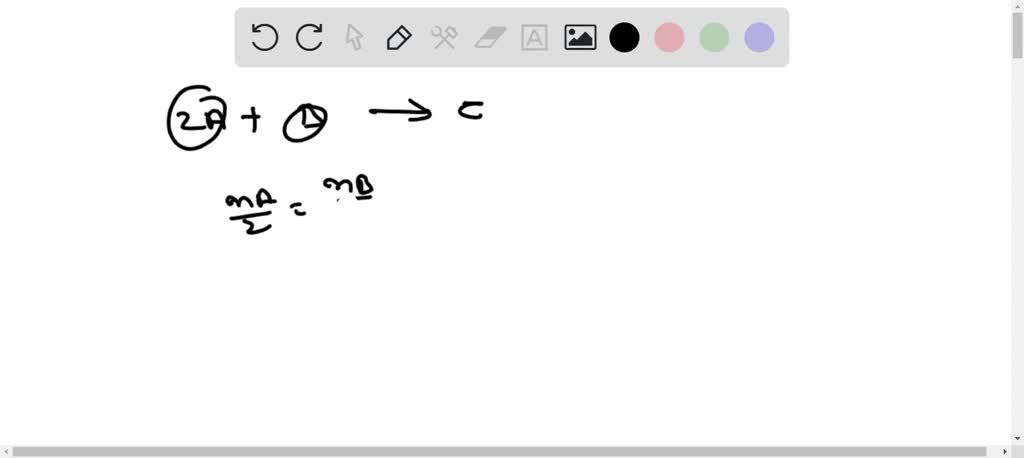
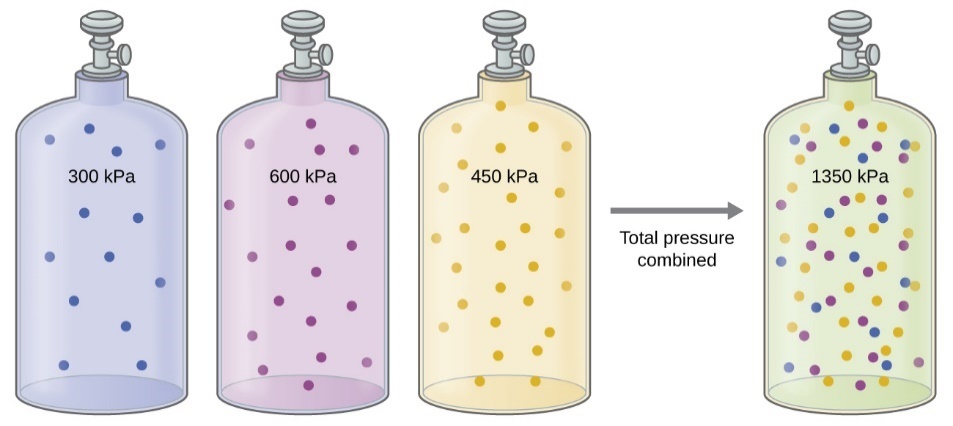
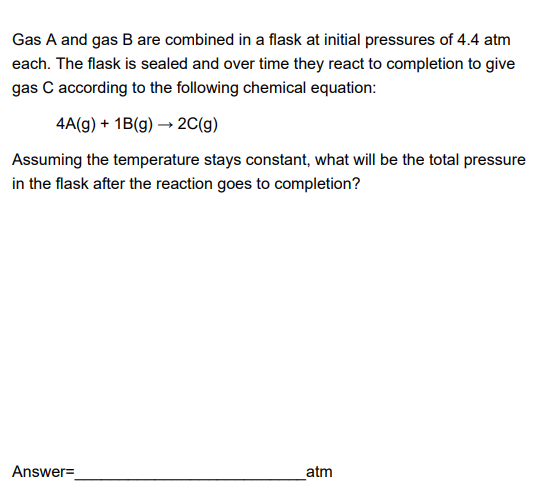
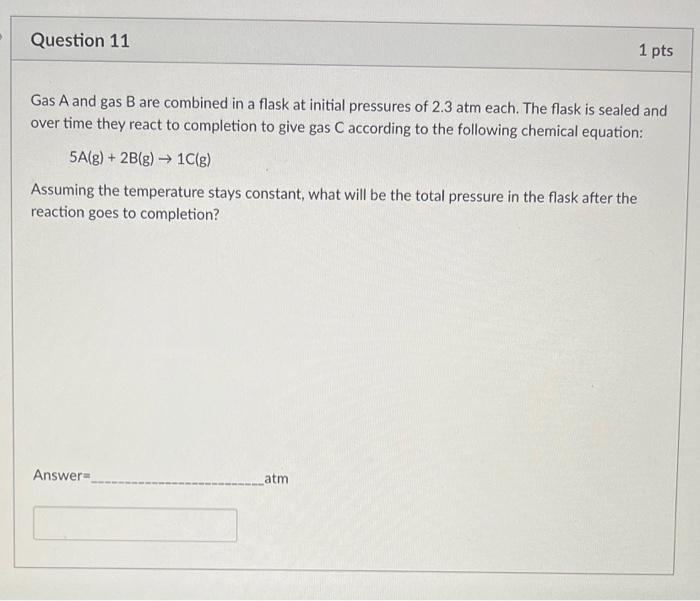
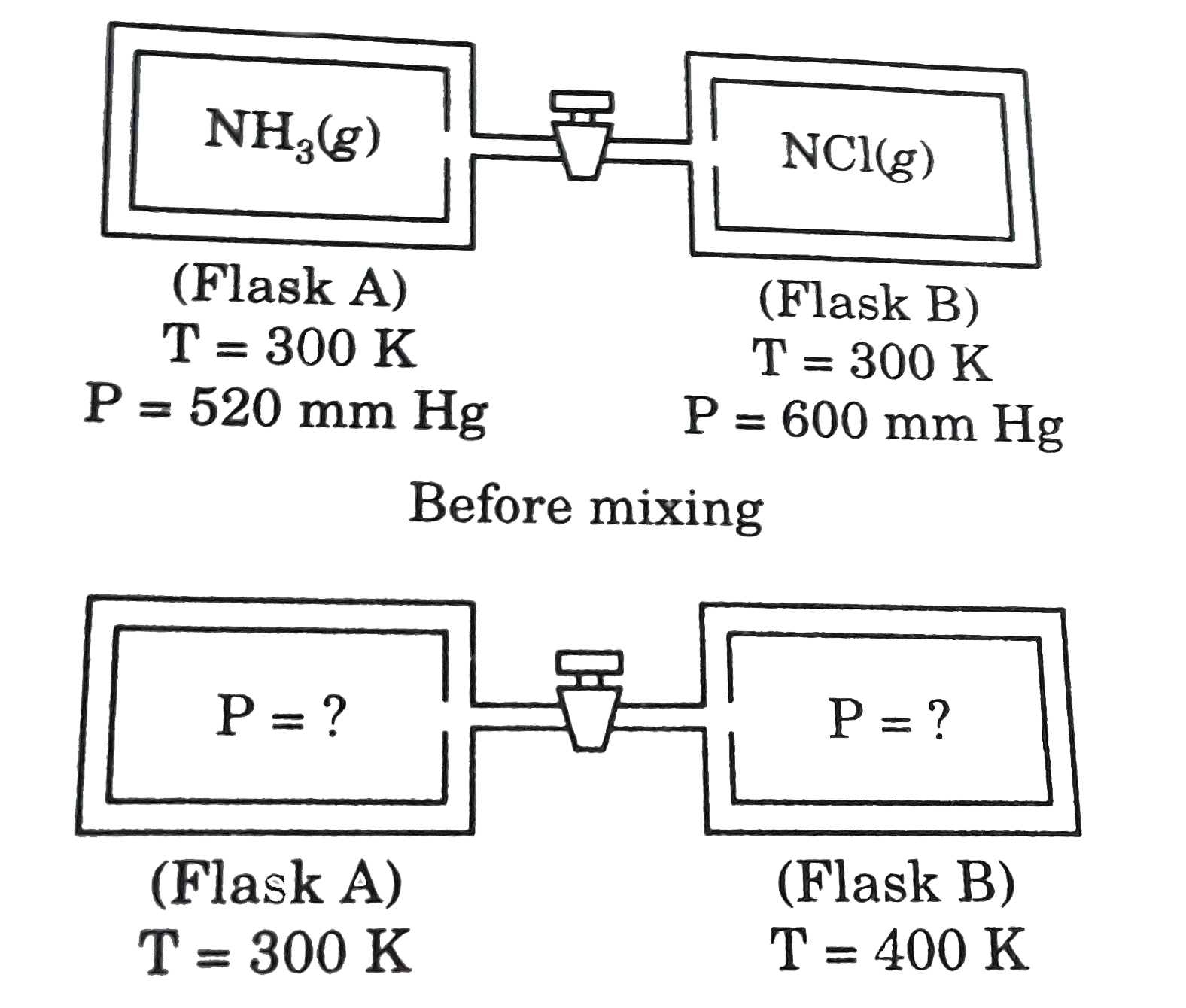
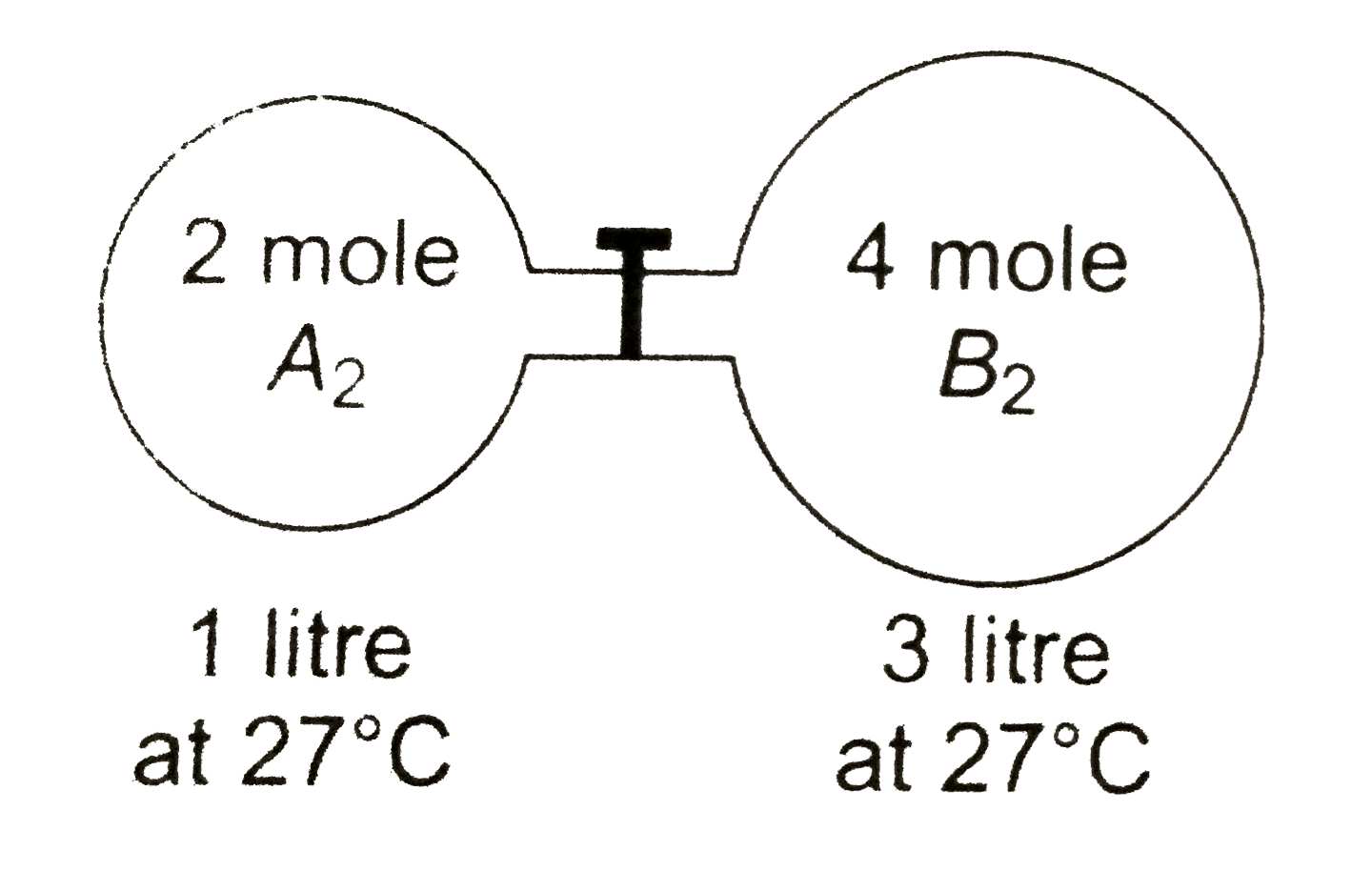Worksheet 3 ch 5 answers.docx - CHM2045: Worksheet 3 ch 5 Dr. Sumner Name You must show your work to display to your TA that you have completed | Course Hero

A 0.5 dm^3 flask contains gas A and 1 dm^3 flask contains gas B at the same temperature. If density of A = 3 g/dm^3 and that of B = 1.5 g/dm^3
A flask contains three times as many moles as H_2 as it does O_2 gas. If hydrogen and oxygen are the only present gases, what is the total pressure flask if the

OneClass: 2.0 L of gas A at 1.0 atm and 1.0 L of gas B at 1.0 atm arecombined in a 3.0 L flask. The f...

SOLVED: Gas A and gas B are combined in a flask at initial pressures of 1.0 atm each. The flask is sealed and over time they react to completion to give gas

SOLVED: 2.0 L of gas A and 1.0 L of gas B are combined in a 3 L flask at 1.0 atm. The flask is sealed and over time they react to
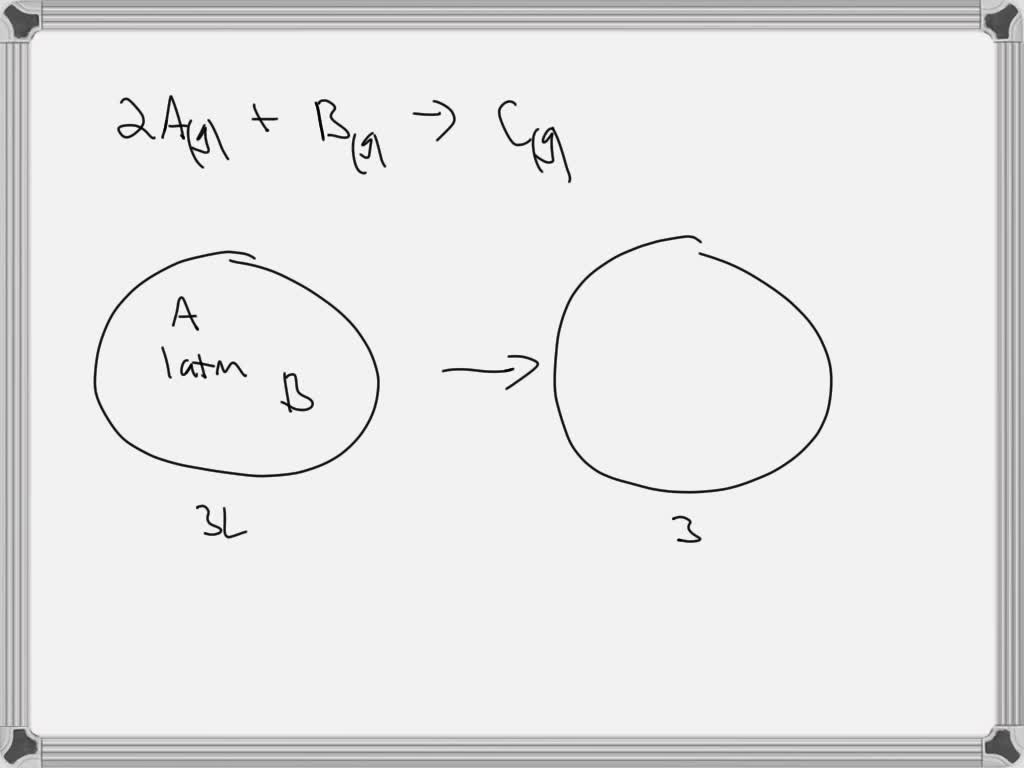
SOLVED: 2.0 L of gas A and 1.0 L of gas B are combined in a 3 L flask at 1.0 atm. The flask is sealed and over time they react to

The gas A2 in the left flask allowed to react with gas B2 present in right flask asA2g+B2g⇌ 2ABg ; KC=4 at 27 ∘ CWhat is the concentration of AB when equilibrium
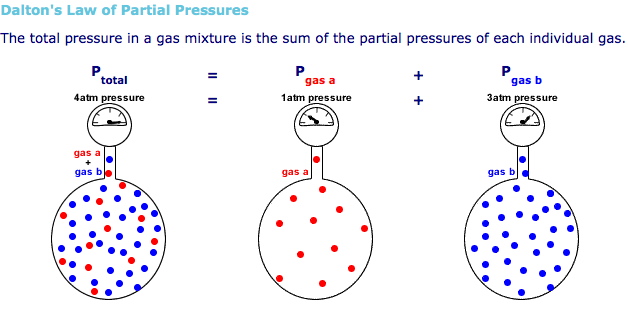
A flask contains a gas mixture of methane, hydrogen. and nitrogen with partial pressures of 1 atm. 1.2 atm, and 1.1 aim, respectively. What is the total pressure of the mixture? | Socratic

Gas absorption release reaction flask. a. 20% of potassium hydroxide... | Download Scientific Diagram

Pressure of 1 g of an ideal gas A at 27 ^∘C is found to be 2 bar. When 2 g of another ideal gas B is introduced in the same flask

Pressure of 1 g of an ideal gas A at 27∘C is found to be 2 bar. When 2 g of another ideal gas B.. - YouTube

SOLVED: Gas A and gas B are combined in a flask at initial pressures of 1.0 atm each. The flask is sealed and over time they react to completion to give gas
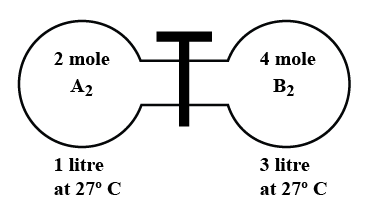
The gas ${{A}_{2}}$ in the left flask allowed to react with gas ${{B}_{2}}$ present in right flask at${{A}_{2}}(g)+{{B}_{2}}(g)\\rightleftharpoons 2AB(g),\\text{ }{{\\text{K}}_{c}}=4$ at ${{27}^{\\circ }}C$ What is the concentration of AB when ...